Moralo oa projeke ea betri ea lithium ion
Lithium-ion battery is an indispensable energy storage product that drives human modern life, Lithium ion batteries are indispensable for daily communication, energy storage, household appliances, electric vehicles, electric ships, etc. And in special applications such as military, deep sea, and mining, lithium-ion batteries are characterized by their high energy density, long service life, and superior safety performance, Widely replaced the traditional lead-acid batteries, nickel-metal hydride batteries and other previous generation products.
Libetri tsa Lithium-ion ke indasteri e sebelisang theknoloji haholo,Nts'etsopele ea indasteri ena e ka boela ea khanna ka mafolofolo nts'etsopele ea indasteri ea tlhahiso ea lisebelisoa le thepa ea naha. Theknoloji e tsoetseng pele ea li-membrane, theknoloji ea ho kopanya e ikemetseng, sebaka se tlase sa phoka le theknoloji e phahameng ea moea e hloekileng le lisebelisoa tse tsamaellanang tse sebelisoang molemong oa tlhahiso ea betri ea lithium ion, E na le tataiso e ntle le karolo ea pontšo bakeng sa nts'etsopele ea mahlale a mang a indasteri le lisebelisoa. Lisebelisoa tse sebelisoang ka libeteri tsa lithium-ion li ka kopanngoa le ore ea lehae ea koporo, cobalt ore le graphite ore bakeng sa ts'ebetso e tebileng ho foil ea koporo e tšesaane haholo bakeng sa libeteri tsa lithium-ion, lithium cobaltate (lithium nickel cobalt manganate) lisebelisoa tsa cathode, le lisebelisoa tse phahameng- lisebelisoa tsa anode tsa matla a graphite.
Le hoja libeteri tsa lithium-ion hona joale ke mofuta o tummeng ka ho fetisisa oa betri, ka tsoelo-pele ea theknoloji, ho na le mefuta e mengata ea libeteri tsa lithium-ion, e 'ngoe le e' ngoe e na le melemo le melemo ea eona. Leha ho le joalo, ka kakaretso, libeteri tsa lithium-ion li na le melemo le mathata a latelang a tloaelehileng:
- Melemo ea libeteri tsa lithium-ion.
(1) Matla a phahameng a matla: Matla a sebetsang a betri e le 'ngoe a ka fihla holimo ho 3.7-3.8V (matla a phahameng ka ho fetisisa a sele a ka lefisoa ho fihlela ho 4.2V), e leng makhetlo a mararo a betri ea Ni Cd le Ni-H.
(2) Matla a phahameng a khethehileng: Hona joale, matla a sebele a tobileng a ka finyelloang a ka bang 555Wh / kg, e bolelang hore thepa e ka finyella matla a itseng a fetang 150mAh / g (3-4 linako tsa Ni Cd, 2-3). makhetlo a Ni MH), e leng haufi le hoo e ka bang 88% ea bohlokoa ba eona ba khopolo.
(3) Bophelo ba nako e telele ea potoloho: ka kakaretso e ka fihla makhetlo a fetang 500, kapa esita le makhetlo a fetang 1000, 'me lithium iron phosphate e ka fihla makhetlo a fetang 2000. Nako ea bophelo ea libeteri tse sebelisang lisebelisoa tse sa hloekang hona joale e tla habeli matla a tsona a tlholisano.
(4) Ts'ebetso e ntle ea polokeho: ha e na tšilafalo, ha e na phello ea mohopolo. Joaloka pele ho Li-ion, libeteri tsa lithium-ion li fokolitse libaka tsa tsona tsa kopo ka lebaka la ho thehoa ha dendrites le lipotoloho tse khutšoanyane tse bakoang ke lithium ea tšepe. Li-ion ha e na lintho tse silafatsang tikoloho joalo ka cadmium, lead, le mercury. Phello e kholo ea libeteri tse ling tsa Ni Cd lits'ebetsong tse itseng (joalo ka sintering) ke "memory effect", e thibelang tšebeliso ea libeteri ka matla. Leha ho le joalo, Li-ion ha e na bothata bona ho hang.
(5) Ho itšehla thajana: Sekhahla sa ho itšehla thajana sa Li ion, se qosoa ka botlalo mocheso oa kamore mme se bolokoa khoeli e le 'ngoe, se ka ba 2%, se tlase haholo ho feta 25-30% ea Ni Cd le 30-35% ea Ni. le MH.
(6) Ho tjhaja le ho tjhaja ka potlako: Bokhoni bo ka fihla ho feta 80% ea matla ka mor'a metsotso e 30 ea ho tjhaja, 'me joale libeteri tsa tšepe tsa phosphate li ka fihla ho 90% ea matla ka mor'a metsotso e 10 ea ho tjhaja.
(7) High working temperature range: The working temperature is -25~55 ° C. With the improvement of the electrolyte and positive electrode, it is expected to expand to -40~70 ° C.
- Melemo ea libeteri tsa lithium-ion
(1) Botsofali: Ho fapana le libeteri tse ling tse ka nchafatsoang, matla a libeteri tsa lithium-ion a tla fokotseha butle, a sa ipapise le palo ea tšebeliso, empa a amana le mocheso. Mokhoa o ka khonehang ke hore khanyetso ea ka hare e eketseha butle-butle, kahoo ho ka etsahala hore e bonahatsoe ka lihlahisoa tsa elektronike tse nang le maqhubu a phahameng a sebetsang. Ho nkela graphite sebaka ka lithium titanate ho bonahala ho lelefatsa bophelo.
(2) Ho se khone ho jara overcharging: Nakong ea ho tjhaja, li-ion tsa lithium tse kentsoeng ka ho fetelletseng li tla lokisoa ka ho sa feleng ka har'a letlapa mme li ke ke tsa lokolloa, tse ka lebisang ho bophelo bo bokhutšoaane ba betri le tlhahiso ea khase e bakang li-bubble tsa khase.
(3) Ho se khone ho mamella ho feta ho tsoa: Nakong ea ho tsoa ho feta tekano, li-ion tsa lithium tse feteletseng li tlosoa ho electrode, tse ka bakang ho putlama ha lattice, ho khutsufatsa nako ea bophelo, le ho baka ho hlahisa khase, ho fella ka li-bubble tsa khase.
(4) Ho hlokahala mekhoa e mengata ea tšireletso: Ka lebaka la hore tšebeliso e fosahetseng e ka fokotsa nako ea bophelo esita le ho lebisa ho phatloha, mekhoa e mengata ea tšireletso e kenyelelitsoe ho moralo oa libeteri tsa lithium-ion.
Potoloho ea ts'ireletso: thibela ho tjhaja ho feta tekano, ho tsoa ho feta tekano, moroalo o mongata, le ho chesa haholo.
Exhaust port: ho thibela khatello e feteletseng ka har'a betri.
Litšobotsi tsa diaphragm: E na le khanyetso e phahameng ea ho phunya ho thibela lipotoloho tse khutšoane tsa kahare; Ha mocheso o ka hare oa betri o phahame haholo, o ntse o ka qhibiliha, o thibela li-ion tsa lithium ho feta, thibela liketso tsa betri, 'me o eketsa ho hanyetsa ka hare (ho fihlela ho 2kQ).
Ka kakaretso, nts'etsopele ea indasteri ea betri ea lithium-ion ke indasteri e matla e khothalletsang linaha tse tsoelang pele ho ea linaheng tse tsoetseng pele haholo.
 |
 |
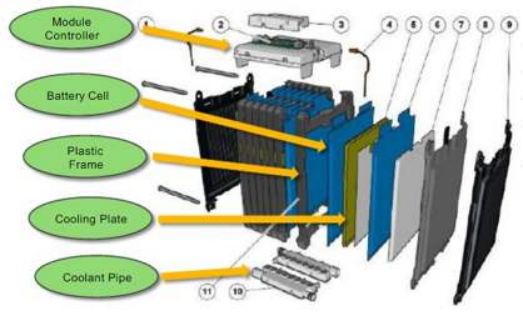 |
 |
Arolelana
-
Lithium Battery Welding Machine | High-Precision, Fast, SafeLitabaNov.17,2025
-
Aluminium Guide Roller | Anodized, Lightweight, Low-NoiseLitabaNov.17,2025
-
Tofu Cat Litter Bulk – Eco, Low-Dust, Fast Clumping SupplyLitabaNov.17,2025
-
Equipment for Lithium Cell Assembly | Automated & PreciseLitabaNov.10,2025
-
Square File Tool – Precision Cut, Hardened Steel, VersatileLitabaNov.10,2025
-
Lithium Ion Battery Assembly Machine | Automated, High-SpeedLitabaNov.10,2025